This chapter addresses three main forms of isomerism: structural, optical, and geometric isomerism. Isomers are molecules that have the same chemical formula but different structures. A structural isomer, also known as a constitutional isomer, is one in which two or more organic compounds have the same molecular formulas but different connectivity of atoms. You will also learn to identify optical isomers, which occur when stereocenters, or tetrahedral carbons with four different substituent groups, are present in a molecule. Enantiomers can be differentiated by the configuration of substituent groups around their stereocenters. Cis-trans (geometric) isomerism exists when there is restricted rotation in a molecule and there are two nonidentical groups on each doubly bonded carbon atom.
20.1 Constitutional Isomers
Learning Objectives
The objective of this section is to introduce you to constitutional isomers using alkanes.
Evaluate the two molecules below.

Here are two alkanes for comparison. How are they the same? How are they different?
Two molecules which have the same molecular formula but different structural formulas, or bonding arrangements, are known as constitutional isomers.
Pentane is an alkane with five carbon atoms. It has three constitutional isomers, shown below.

Unbranched isomer of pentane | 
Branched constitutional isomer of pentane | 
Branched constitutional isomer of pentane |
Practice Questions
- Draw three constitutional isomers of heptane.
Circle the constitutional isomers of hexane in the figure below. Why are the remaining molecules not considered to be constitutional isomers of hexane?

- Draw the constitutional isomer of hexane that is missing.
- What is the minimum number of carbons in the chain of an alkane to be able to have a constitutional isomer?
Citation of Previous Version
O’Donnell, R. (2020). Constitutional Isomers. In Organic Chemistry Nomenclature Workbook (O’Donnell). LibreTexts. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemistry_Nomenclature_Workbook_(O’Donnell)/01%3A_Chapters/1.02%3A_Constitutional_Isomers
20.2 The Reason for Handedness in Molecules - Chirality
Learning Objectives
By the end of this section, you will be able to:
- Determine whether or not a compound is chiral, given its Kekulé, condensed or shorthand structure, with or without the aid of molecular models.
- Label the chiral centres (carbon atoms) in a given Kekulé, condensed or shorthand structure.
Symmetry and Chirality
Molecules that are nonsuperimposable mirror images of each other are said to be chiral (pronounced “ky-ral,” from the Greek cheir, meaning “hand”). Examples of some familiar chiral objects are your hands. Your left and right hands are nonsuperimposable mirror images. (Try putting your right shoe on your left foot—it just doesn’t work.) An achiral object is one that can be superimposed on its mirror image, as shown by the superimposed flasks in the figure below.
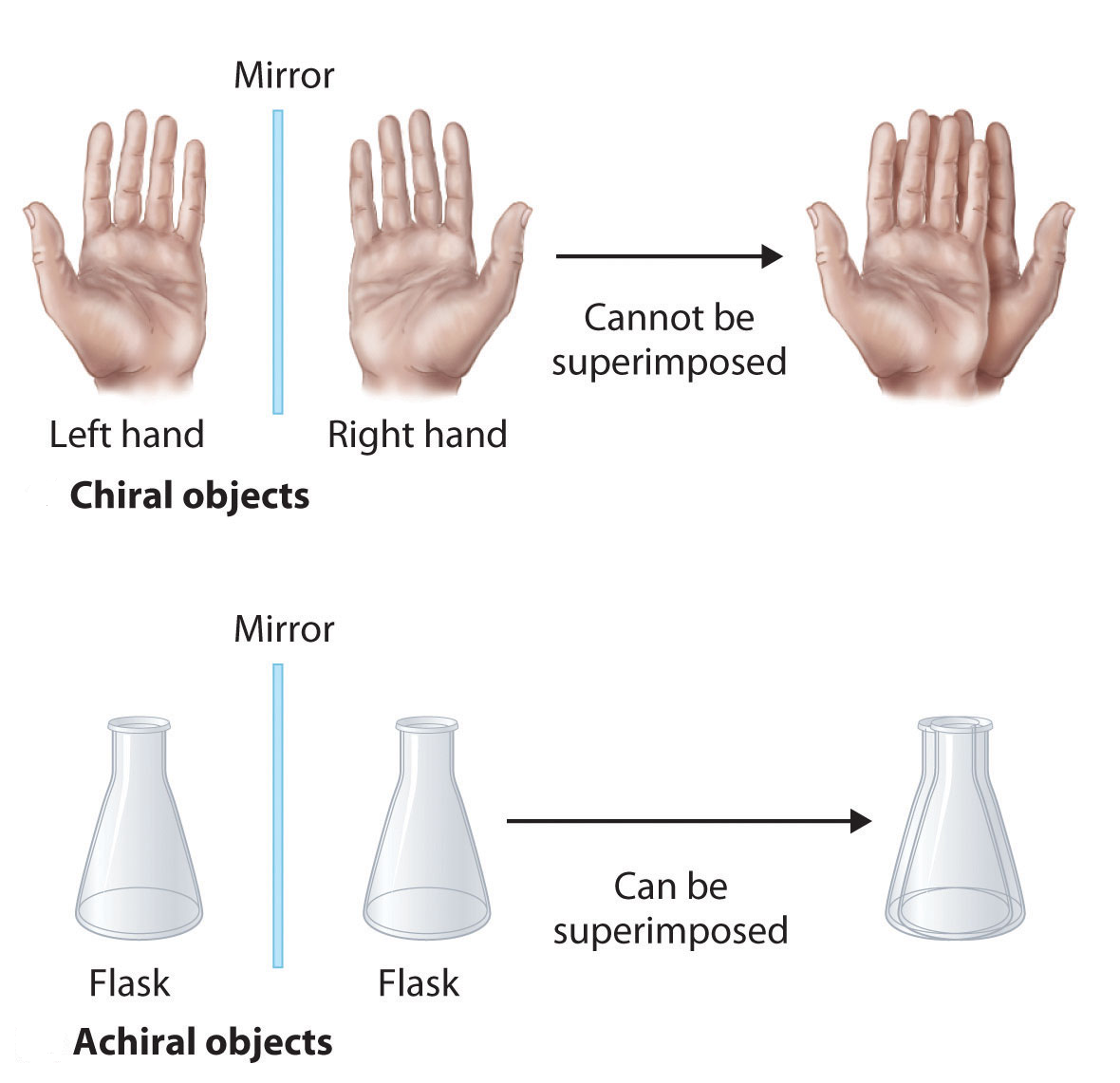
An an important questions is why is one chiral and the other not? The answer is that the flask has a plane of symmetry and your hand does not. A plane of symmetry is a plane or a line through an object which divides the object into two halves that are mirror images of each other. When looking at the flask, a line can be drawn down the middle which separates it into two mirror image halves. However, a similar line down the middle of a hand separates it into two non-mirror image halves. This idea can be used to predict chirality. If an object or molecule has a plane of symmetry it is achiral. If if lacks a plane of symmetry it is chiral.

Symmetry can be used to explain why a carbon bonded to four different substituents is chiral. When a carbon is bonded to fewer than four different substituents it will have a plane of symmetry making it achiral. A carbon atom that is bonded to four different substituents loses all symmetry, and is often referred to as an asymmetric carbon. The lack of a plane of symmetry makes the carbon chiral. The presence of a single chiral carbon atom sufficient to render the molecule chiral, and modern terminology refers to such groupings as chiral centers or stereo centers.
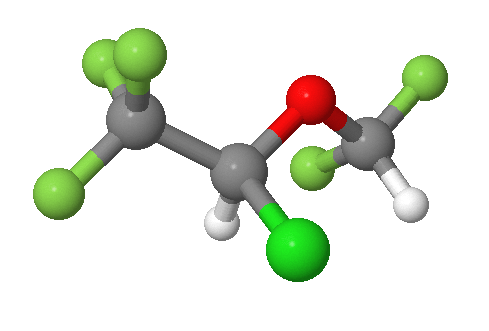
An example is shown in the bromochlorofluoromethane molecule shown in part (a) of the figure below. This carbon, is attached to four different substituents making it chiral. which is often designated by an asterisk in structural drawings. If the bromine atom is replaced by another chlorine to make dichlorofluoromethane, as shown in part (b) below, the molecule and its mirror image can now be superimposed by simple rotation. Thus the carbon is no longer a chiral center. Upon comparison, bromochlorofluoromethane lacks a plane of symmetry while dichlorofluoromethane has a plane of symmetry.


_and_bromochlorofluoromethane_(does_not_have_a_plane_of_symmetry%252C_so_achiral).svg?revision=1&size=bestfit&width=359&height=149)
Identifying Chiral carbons
Identifying chiral carbons in a molecule is an important skill for organic chemists. The presence of a chiral carbon presents the possibility of a molecule having multiple stereoisomers. Most of the chiral centers we shall discuss in this chapter are asymmetric carbon atoms, but it should be recognized that other tetrahedral or pyramidal atoms may become chiral centers if appropriately substituted. Also, when more than one chiral center is present in a molecular structure, care must be taken to analyze their relationship before concluding that a specific molecular configuration is chiral or achiral. This aspect of stereoisomerism will be treated later. Because an carbon requires four different substituents to become asymmertric, it can be said, with few exceptions, that sp2 and sp hybridized carbons involved in multiple bonds are achiral. Also, any carbon with more than one hydrogen, such as a -CH3 or -CH2- group, are also achiral.
Looking for planes of symmetry in a molecule is useful, but often difficult in practice. It is difficult to illustrate on the two dimensional page, but you will see if you build models of these achiral molecules that, in each case, there is at least one plane of symmetry, where one side of the plane is the mirror image of the other. In most cases, the easiest way to decide whether a molecule is chiral or achiral is to look for one or more stereocenters - with a few rare exceptions, the general rule is that molecules with at least one stereocenter are chiral, and molecules with no stereocenters are achiral.
Determining if a carbon is bonded to four distinctly different substituents can often be difficult to ascertain. Remember even the slightest difference makes a substituent unique. Often these difference can be distant from the chiral carbon itself. Careful consideration and often the building of molecular models may be required. A good example is shown below. It may appear that the molecule is achiral, however, when looking at the groups directly attached to the possible chiral carbon, it is clear that they all different. The two alkyl groups are differ by a single -CH2- group which is enough to consider them different.

Predict if the following molecule would be chiral or achiral:

Achiral. When determining the chirality of a molecule, it best to start by locating any chiral carbons. An obvious candidate is the ring carbon attached to the methyl substituent. The question then becomes: does the ring as two different substituents making the substituted ring carbon chiral? With an uncertainty such as this, it is then helpful try to identify any planes of symmetry in the molecule. This molecule does have a plane of symmetry making the molecule achiral. The plane of symmetry would be easier see if the molecule were view from above. Typically, monosubstituted cycloalkanes have a similar plane of symmetry making them all achiral.

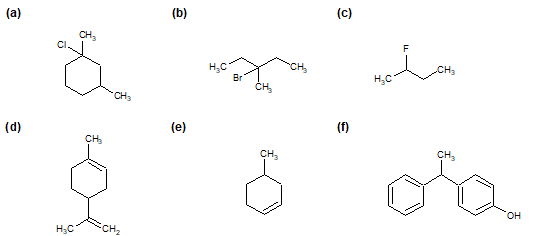
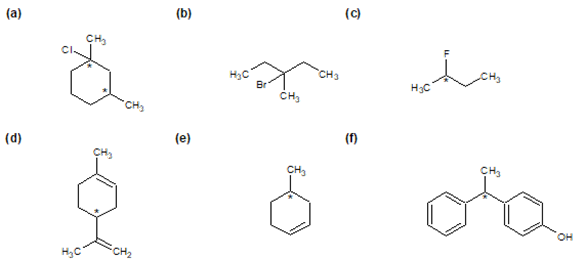
Determine if each of the following molecules are chiral or achiral. For chiral molecules indicate any chiral carbons.


Explanation
Structures F and G are achiral. The former has a plane of symmetry passing through the chlorine atom and bisecting the opposite carbon-carbon bond. The similar structure of compound E does not have such a symmetry plane, and the carbon bonded to the chlorine is a chiral center (the two ring segments connecting this carbon are not identical). Structure G is essentially flat. All the carbons except that of the methyl group are sp2 hybridized, and therefore trigonal-planar in configuration. Compounds C, D & H have more than one chiral center, and are also chiral.
Something Extra
In the 1960’s, a drug called thalidomide was widely prescribed in the Western Europe to alleviate morning sickness in pregnant women.

Thalidomide had previously been used in other countries as an antidepressant, and was believed to be safe and effective for both purposes. The drug was not approved for use in the U.S.A. It was not long, however, before doctors realized that something had gone horribly wrong: many babies born to women who had taken thalidomide during pregnancy suffered from severe birth defects.
Researchers later realized the problem lay in the fact that thalidomide was being provided as a mixture of two different isomeric forms.

One of the isomers is an effective medication, the other caused the side effects. Both isomeric forms have the same molecular formula and the same atom-to-atom connectivity, so they are not constitutional isomers. Where they differ is in the arrangement in three-dimensional space about one tetrahedral, sp3-hybridized carbon. These two forms of thalidomide are stereoisomers. If you make models of the two stereoisomers of thalidomide, you will see that they too are mirror images, and cannot be superimposed.

As a historical note, thalidomide was never approved for use in the United States. This was thanks in large part to the efforts of Dr. Frances Kelsey, a Food and Drug officer who, at peril to her career, blocked its approval due to her concerns about the lack of adequate safety studies, particularly with regard to the drug's ability to enter the bloodstream of a developing fetus. Unfortunately, though, at that time clinical trials for new drugs involved widespread and unregulated distribution to doctors and their patients across the country, so families in the U.S. were not spared from the damage caused.
Very recently a close derivative of thalidomide has become legal to prescribe again in the United States, with strict safety measures enforced, for the treatment of a form of blood cancer called multiple myeloma. In Brazil, thalidomide is used in the treatment of leprosy - but despite safety measures, children are still being born with thalidomide-related defects.
Label the molecules below as chiral or achiral, and locate all stereocenters.

1) For the following compounds, star (*) each chiral center, if any.
2) Explain why the following compound is chiral.

3) Determine which of the following objects is chiral.
a) A Glove.
b) A nail.
c) A pair of sunglasses.
d) The written word "Chiral".
4) Place an "*" by all of the chrial carbons in the following molecules.
a)
Erythrose, a four carbon sugar.

b) Isoflurane, an anestetic. Bright green = Chlorine, Pale green = Fluorine.
1)
2) Though the molecule does not contain a chiral carbon, it is chiral as it is non-superimposable on its mirror image due to its twisted nature (the twist comes from the structure of the double bonds needing to be at 90° angles to each other, preventing the molecule from being planar).
3)
a) Just as hands are chiral a glove must also be chiral.
b) A nail has a plane of symmetry which goes down the middle making it a achiral.
c) A pair of sunglasses has a plane of symmetry which goes through the nose making it achiral.
d) Most written words are chiral. Look one in a mirror to confirm this.
4
a)

b)

Circle all of the carbon stereocenters in the molecules below.

Circle all of the carbon stereocenters in the molecules below.

Here are some more examples of chiral molecules that exist as pairs of enantiomers. In each of these examples, there is a single stereocenter, indicated with an arrow. (Many molecules have more than one stereocenter, but we will get to that that a little later!)

Here are some examples of molecules that are achiral (not chiral). Notice that none of these molecules has a stereocenter.

It is difficult to illustrate on the two dimensional page, but you will see if you build models of these achiral molecules that, in each case, there is at least one plane of symmetry, where one side of the plane is the mirror image of the other. Chirality is tied conceptually to the idea of asymmetry, and any molecule that has a plane of symmetry cannot be chiral. When looking for a plane of symmetry, however, we must consider all possible conformations that a molecule could adopt. Even a very simple molecule like ethane, for example, is asymmetric in many of its countless potential conformations – but it has obvious symmetry in both the eclipsed and staggered conformations, and for this reason it is achiral.
Looking for planes of symmetry in a molecule is useful, but often difficult in practice. In most cases, the easiest way to decide whether a molecule is chiral or achiral is to look for one or more stereocenters - with a few rare exceptions (see section 3.7B), the general rule is that molecules with at least one stereocenter are chiral, and molecules with no stereocenters are achiral. Carbon stereocenters are also referred to quite frequently as chiral carbons.
When evaluating a molecule for chirality, it is important to recognize that the question of whether or not the dashed/solid wedge drawing convention is used is irrelevant. Chiral molecules are sometimes drawn without using wedges (although obviously this means that stereochemical information is being omitted). Conversely, wedges may be used on carbons that are not stereocenters – look, for example, at the drawings of glycine and citrate in the figure above. Just because you see dashed and solid wedges in a structure, do not automatically assume that you are looking at a stereocenter.
Other elements in addition to carbon can be stereocenters. The phosphorus center of phosphate ion and organic phosphate esters, for example, is tetrahedral, and thus is potentially a stereocenter.
%252C_phosphate_ester_(chiral)%252C_and_phosphate_triester_(chiral)._.svg?revision=1&size=bestfit&width=421&height=103)
We will see in chapter 10 how researchers, in order to investigate the stereochemistry of reactions at the phosphate center, incorporated sulfur and/or 17O and 18O isotopes of oxygen (the ‘normal’ isotope is 16O) to create chiral phosphate groups. Phosphate triesters are chiral if the three substituent groups are different.
Asymmetric quaternary ammonium groups are also chiral. Amines, however, are not chiral, because they rapidly invert, or turn ‘inside out’, at room temperature.

Label the molecules below as chiral or achiral, and circle all stereocenters.
a) fumarate (a citric acid cycle intermediate)

b) malate (a citric acid cycle intermediate)

b) malate (a citric acid cycle intermediate)

a) achiral (no stereocenters)

b) chiral

c) chiral

Label the molecules below as chiral or achiral, and circle all stereocenters.
a) acetylsalicylic acid (aspirin)

b) acetaminophen (active ingredient in Tylenol)

c) thalidomide (drug that caused birth defects in pregnant mothers in the 1960’s)

a) achiral (no stereocenters)

b) achiral (no stereocenters)

c) chiral

Draw both enantiomers of the following chiral amino acids.
a) Cysteine 
b) Proline 
Draw both enantiomers of the following compounds from the given names.
a) 2-bromobutane
b) 2,3-dimethyl-3-pentanol
Which of the following body parts are chiral?
a) Hands b) Eyes c) Feet d) Ears
a) Hands- chiral since the mirror images cannot be superimposed (think of the example in the beginning of the section)
b) Eyes- achiral since mirror images that are superimposable
c) Feet- chiral since the mirror images cannot be superimposed (Does your right foot fit in your left shoe?)
d) Ears- chiral since the mirror images cannot be superimposed
Circle the chiral centers in the following compounds.

Identify the chiral centers in the following compounds.

Citation of Previous Version
Kennepohl, D., Farmer, S., Reusch, W., Soderberg, T., Clark, J., Sharrett, Z., Morsch, L., & Cunningham, K. (2022). The Reason for Handedness in Molecules - Chirality. In Organic Chemistry (McMurry). LibreTexts. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/05%3A_Stereochemistry_at_Tetrahedral_Centers/5.02%3A_The_Reason_for_Handedness_in_Molecules_-_Chirality
20.3 Cis-Trans Isomers (Geometric Isomers)
Learning Objectives
By the end of this section, you will be able to:
- Recognize that alkenes that can exist as cis-trans isomers.
- Classify isomers as cis or trans.
- Draw structures for cis-trans isomers given their names.
There is free rotation about the carbon-to-carbon single bonds (C–C) in alkanes. In contrast, the structure of alkenes requires that the carbon atoms of a double bond and the two atoms bonded to each carbon atom all lie in a single plane, and that each doubly bonded carbon atom lies in the center of a triangle. This part of the molecule’s structure is rigid; rotation about doubly bonded carbon atoms is not possible without rupturing the bond. Look at the two chlorinated hydrocarbons in the figure below.

Table 20.1: Rotation about Bonds. In 1,2-dichloroethane (a), free rotation about the C–C bond allows the two structures to be interconverted by a twist of one end relative to the other. In 1,2-dichloroethene (b), restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond are significant.
In 1,2-dichloroethane (part (a) of the figure above), there is free rotation about the C–C bond. The two models shown represent exactly the same molecule; they are not isomers. You can draw structural formulas that look different, but if you bear in mind the possibility of this free rotation about single bonds, you should recognize that these two structures represent the same molecule:

In 1,2-dichloroethene, however, restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond become significant. This leads to a special kind of isomerism. The isomer in which the two chlorine (Cl) atoms lie on the same side of the molecule is called the cis isomer (Latin cis, meaning “on this side”) and is named cis-1,2-dichloroethene. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer (Latin trans, meaning “across”) and is named trans-1,2-dichloroethene. These two compounds are cis-trans isomers (or geometric isomers), compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule.
Consider the alkene with the condensed structural formula CH3CH=CHCH3. We could name it 2-butene, but there are actually two such compounds; the double bond results in cis-trans isomerism.
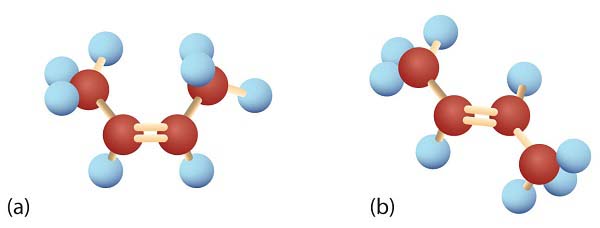 Ball-and-Spring Models of (a) Cis-2-Butene and (b) Trans-2-Butene. Cis-trans isomers have different physical, chemical, and physiological properties.
Ball-and-Spring Models of (a) Cis-2-Butene and (b) Trans-2-Butene. Cis-trans isomers have different physical, chemical, and physiological properties.Cis-2-butene has both methyl groups on the same side of the molecule. Trans-2-butene has the methyl groups on opposite sides of the molecule. Their structural formulas are as follows:
Models of (left) Cis-2-Butene and (right) Trans-2-Butene.
Note, however, that the presence of a double bond does not necessarily lead to cis-trans isomerism. We can draw two seemingly different propenes:
Different views of the propene molecule (flip vertically). These are not isomers.
However, these two structures are not really different from each other. If you could pick up either molecule from the page and flip it over top to bottom, you would see that the two formulas are identical. Thus there are two requirements for cis-trans isomerism:
- Rotation must be restricted in the molecule.
- There must be two nonidentical groups on each doubly bonded carbon atom.
In these propene structures, the second requirement for cis-trans isomerism is not fulfilled. One of the doubly bonded carbon atoms does have two different groups attached, but the rules require that both carbon atoms have two different groups. In general, the following statements hold true in cis-trans isomerism:
- Alkenes with a C=CH2 unit do not exist as cis-trans isomers.
- Alkenes with a C=CR2 unit, where the two R groups are the same, do not exist as cis-trans isomers.
- Alkenes of the type R–CH=CH–R can exist as cis and trans isomers; cis if the two R groups are on the same side of the carbon-to-carbon double bond, and trans if the two R groups are on opposite sides of the carbon-to-carbon double bond.
Advanced Note: E/Z Isomerization
If a molecule has a C=C bond with one non-hydrogen group attached to each of the carbons, cis/trans nomenclature descried above is enough to describe it. However, if you have three different groups (or four), then the cis/trans approach is insufficient to describe the different isomers, since we do not know which two of the three groups are being described. For example, if you have a C=C bond, with a methyl group and a bromine on one carbon , and an ethyl group on the other, it is neither trans nor cis, since it is not clear whether the ethyl group is trans to the bromine or the methyl. This is addressed with a more advanced E/Z nomenclature discussed elsewhere.
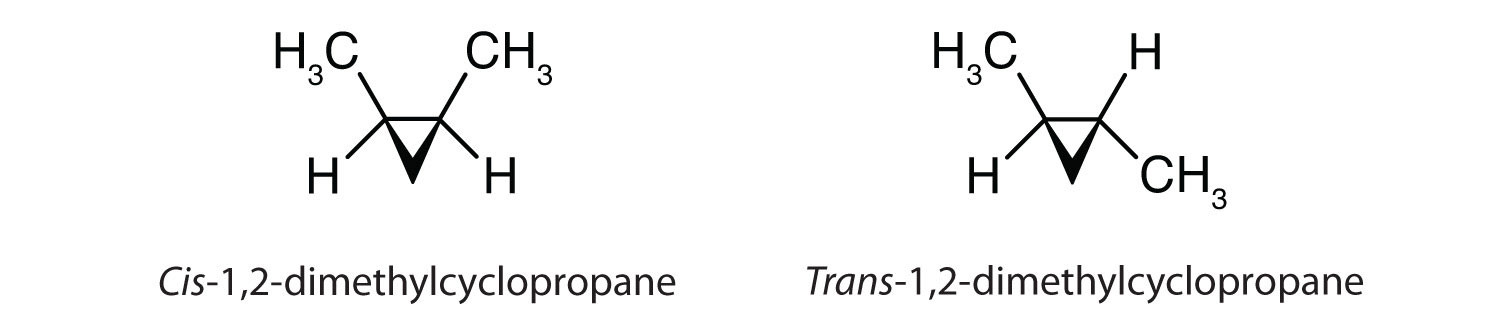
Cis-trans isomerism also occurs in cyclic compounds. In ring structures, groups are unable to rotate about any of the ring carbon–carbon bonds. Therefore, groups can be either on the same side of the ring (cis) or on opposite sides of the ring (trans). For our purposes here, we represent all cycloalkanes as planar structures, and we indicate the positions of the groups, either above or below the plane of the ring.
Which compounds can exist as cis-trans (geometric) isomers? Draw them.
- CHCl=CHBr
- CH2=CBrCH3
- (CH3)2C=CHCH2CH3
- CH3CH=CHCH2CH3
All four structures have a double bond and thus meet rule 1 for cis-trans isomerism.
This compound meets rule 2; it has two nonidentical groups on each carbon atom (H and Cl on one and H and Br on the other). It exists as both cis and trans isomers:
- This compound has two hydrogen atoms on one of its doubly bonded carbon atoms; it fails rule 2 and does not exist as cis and trans isomers.
- This compound has two methyl (CH3) groups on one of its doubly bonded carbon atoms. It fails rule 2 and does not exist as cis and trans isomers.
This compound meets rule 2; it has two nonidentical groups on each carbon atom and exists as both cis and trans isomers:
What are cis-trans (geometric) isomers? What two types of compounds can exhibit cis-trans isomerism?
Classify each compound as a cis isomer, a trans isomer, or neither.
Cis-trans isomers are compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule. Alkenes and cyclic compounds can exhibit cis-trans isomerism.
- trans (the two hydrogen atoms are on opposite sides)
- cis (the two hydrogen atoms are on the same side, as are the two ethyl groups)
- cis (the two ethyl groups are on the same side)
- neither (fliping the bond does not change the molecule. There are no isomers for this molecule)
Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.
- 2-bromo-2-pentene
- 3-hexene
- 4-methyl-2-pentene
- 1,1-dibromo-1-butene
- 2-butenoic acid (CH3CH=CHCOOH)
Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.
- 2,3-dimethyl-2-pentene
- 1,1-dimethyl-2-ethylcyclopropane
- 1,2-dimethylcyclohexane
- 5-methyl-2-hexene
- 1,2,3-trimethylcyclopropane
a: none. There are two distinct geometric isomers, but since there are there are four different groups off the double bond, these are both cis/trans isomers (they are technically E/Z isomers discussed elsewhere).
b:
c: 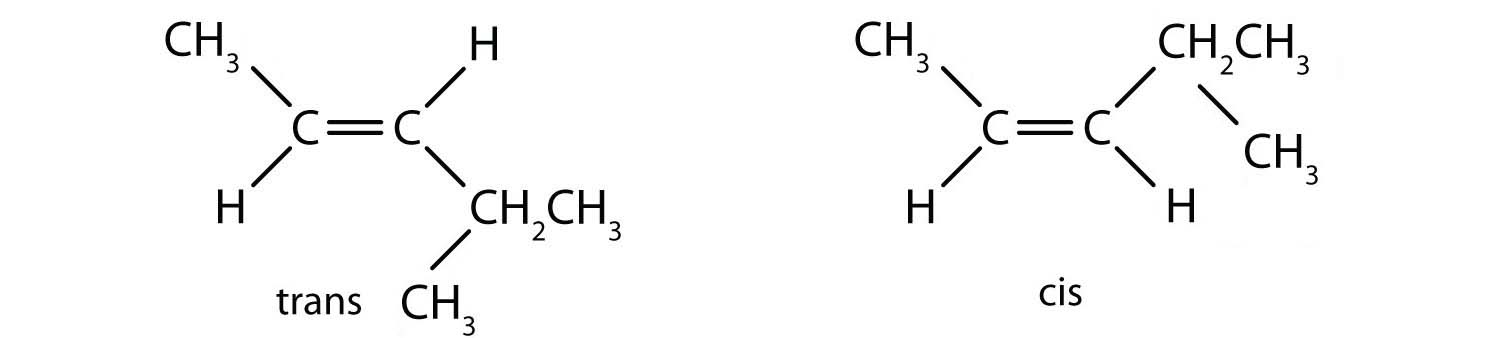
d: 
Citation of Previous Version
LibreTexts. (2022). Cis-Trans Isomers (Geometric Isomers). In CHEM 121: Concepts for a Molecular View of Biology II (Cunningham). LibreTexts. https://chem.libretexts.org/Courses/Case_Western_Reserve_University/CHEM_121%3A_Concepts_for_a_Molecular_View_of_Biology_II_(Cunningham)/1%3A_Organic_Chemistry_Basics/1.11%3A_Cis-Trans_Isomers_(Geometric_Isomers)