In the following sections, many of the common functional groups found in organic chemistry will be described. Tables of these functional groups can be found at the bottom of the page.
Hydrocarbons
The simplest functional group in organic chemistry (which is often ignored when listing functional groups) is called an alkane, characterized by single bonds between two carbons and between carbon and hydrogen. Some examples of alkanes include methane, CH4, is the natural gas you may burn in your furnace or on a stove. Octane, C8H18, is a component of gasoline.
Alkanes

Alkenes (sometimes called olefins) have carbon-carbon double bonds, and alkynes have carbon-carbon triple bonds. Ethene, the simplest alkene example, is a gas that serves as a cellular signal in fruits to stimulate ripening. (If you want bananas to ripen quickly, put them in a paper bag along with an apple - the apple emits ethene gas, setting off the ripening process in the bananas). Ethyne, commonly called acetylene, is used as a fuel in welding blow torches.
Alkenes and alkynes

Alkenes have trigonal planar electron geometry (due to sp2 hybrid orbitals at the alkene carbons) while alkynes have linear geometry (due to sp hybrid orbitals at the alkyne carbons). Furthermore, many alkenes can take two geometric forms: cis or trans (or Z and E which will be explained in detail in Chapter 7). The cis and trans forms of a given alkene are different molecules with different physical properties there is a very high energy barrier to rotation about a double bond. In the example below, the difference between cis and trans alkenes is readily apparent.


Alkanes, alkenes, and alkynes are all classified as hydrocarbons, because they are composed solely of carbon and hydrogen atoms. Alkanes are said to be saturated hydrocarbons, because the carbons are bonded to the maximum possible number of hydrogens - in other words, they are saturated with hydrogen atoms. The double and triple-bonded carbons in alkenes and alkynes have fewer hydrogen atoms bonded to them - they are thus referred to as unsaturated hydrocarbons. Hydrogen can be added to double and triple bonds, in a type of reaction called 'hydrogenation'.
The aromatic group is exemplified by benzene (which used to be a commonly used solvent on the organic lab, but which was shown to be carcinogenic), and naphthalene, a compound with a distinctive 'mothball' smell. Aromatic groups are planar (flat) ring structures, and are widespread in nature.
Aromatics
Functional Groups with Carbon Single Bonds to other Atoms
Halides
When the carbon of an alkane is bonded to one or more halogens, the group is referred to as a alkyl halide or haloalkane. The presence of a halogen atom (F, Cl, Br, or I), is often represented by X due to the similar chemistry of halogens. Chloroform is a useful solvent in the laboratory, and was one of the earlier anesthetic drugs used in surgery. Chlorodifluoromethane was used as a refrigerant and in aerosol sprays until the late twentieth century, but its use was discontinued after it was found to have harmful effects on the ozone layer. Bromoethane is a simple alkyl halide often used in organic synthesis. Alkyl halides groups are quite rare in biomolecules.

Alcohols and Thiols
In the alcohol functional group, a carbon is single-bonded to an OH group (the OH group, by itself, is referred to as a hydroxyl). Except for methanol, all alcohols can be classified as primary, secondary, or tertiary. In a primary alcohol, the carbon bonded to the OH group is also bonded to only one other carbon. In a secondary alcohol and tertiary alcohol, the carbon is bonded to two or three other carbons, respectively. When the hydroxyl group is directly attached to an aromatic ring, the resulting group is called a phenol.

The sulfur analog of an alcohol is called a thiol (the prefix thio, derived from the Greek, refers to sulfur).

Ethers and sulfides
In an ether functional group, a central oxygen is bonded to two carbons. Below are the line and Lewis structures of diethyl ether, a common laboratory solvent and also one of the first medical anaesthesia agents.

In sulfides, the oxygen atom of an ether has been replaced by a sulfur atom.

Amines
Amines are characterized by nitrogen atoms with single bonds to hydrogen and carbon. Just as there are primary, secondary, and tertiary alcohols, there are primary, secondary, and tertiary amines. Ammonia is a special case with no carbon atoms.

One of the most important properties of amines is that they are basic, and are readily protonated to form ammonium cations. In the case where a nitrogen has four bonds to carbon (which is somewhat unusual in biomolecules), it is called a quaternary ammonium ion.


Note: Do not be confused by how the terms 'primary', 'secondary', and 'tertiary' are applied to alcohols and amines - the definitions are different. In alcohols, what matters is how many other carbons the alcohol carbon is bonded to, while in amines, what matters is how many carbons the nitrogen is bonded to.

Carbonyl Containing Functional Groups
Aldehydes and Ketones
There are a number of functional groups that contain a carbon-oxygen double bond, which is commonly referred to as a carbonyl. Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens.

Carboxylic acids and acid derivatives
If a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to a heteroatom (in organic chemistry, this term generally refers to oxygen, nitrogen, sulfur, or one of the halogens), the functional group is considered to be one of the ‘carboxylic acid derivatives’, a designation that describes a grouping of several functional groups. The eponymous member of this grouping is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl (OH) group.

As the name implies, carboxylic acids are acidic, meaning that they are readily deprotonated to form the conjugate base form, called a carboxylate.

In amides, the carbonyl carbon is bonded to a nitrogen. The nitrogen in an amide can be bonded either to hydrogens, to carbons, or to both. Another way of thinking of an amide is that it is a carbonyl bonded to an amine.

In esters, the carbonyl carbon is bonded to an oxygen which is itself bonded to another carbon. Another way of thinking of an ester is that it is a carbonyl bonded to an alcohol. Thioesters are similar to esters, except a sulfur is in place of the oxygen.

In an acid anhydride, there are two carbonyl carbons with an oxygen in between. An acid anhydride is formed from combination of two carboxylic acids with the loss of water (anhydride).

In an acyl phosphate, the carbonyl carbon is bonded to the oxygen of a phosphate, and in an acid chloride, the carbonyl carbon is bonded to a chlorine.

Nitriles and Imines
In a nitrile group, a carbon is triple-bonded to a nitrogen. Nitriles are also often referred to as cyano groups.

Molecules with carbon-nitrogen double bonds are called imines, or Schiff bases.

Phosphates
Phosphorus is a very important element in biological organic chemistry, and is found as the central atom in the phosphate group. Many biological organic molecules contain phosphate, diphosphate, and triphosphate groups, which are linked to a carbon atom by the phosphate ester functionality.

Because phosphates are so abundant in biological organic chemistry, it is convenient to depict them with the abbreviation 'P'. Notice that this 'P' abbreviation includes the oxygen atoms and negative charges associated with the phosphate groups.


Molecules with Multiple Functional Groups
A single compound may contain several different functional groups. The six-carbon sugar molecules glucose and fructose, for example, contain aldehyde and ketone groups, respectively, and both contain five alcohol groups (a compound with several alcohol groups is often referred to as a ‘polyol’).


Capsaicin, the compound responsible for the heat in hot peppers, contains phenol, ether, amide, and alkene functional groups.

The male sex hormone testosterone contains ketone, alkene, and secondary alcohol groups, while acetylsalicylic acid (aspirin) contains aromatic, carboxylic acid, and ester groups.


While not in any way a complete list, this section has covered most of the important functional groups that we will encounter in biological and laboratory organic chemistry. The table found below provides a summary of all of the groups listed in this section, plus a few more that will be introduced later in the text.
Identify the functional groups in the following organic compounds. State whether alcohols and amines are primary, secondary, or tertiary.
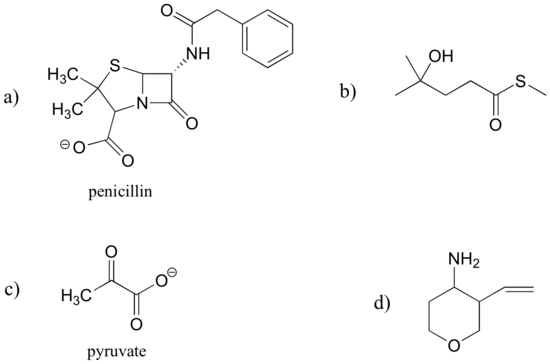
a) carboxylate, sulfide, aromatic, two amide groups (one of which is cyclic)
b) tertiary alcohol, thioester
c) carboxylate, ketone
d) ether, primary amine, alkene
The following is the molecule for ATP, or the molecule responsible for energy in human cells. Identify the functional groups for ATP.
