Isotopes of an element are atoms with the same atomic number but different mass numbers; isotopes of an element, therefore, differ from each other only in the number of neutrons within the nucleus. When a naturally occurring element is composed of several isotopes, the atomic mass of the element represents the average of the masses of the isotopes involved. A chemical symbol identifies the atoms in a substance using symbols, which are one-, two-, or three-letter abbreviations for the atoms. The discovery of the periodic recurrence of similar properties among the elements led to the formulation of the periodic table, in which the elements are arranged in order of increasing atomic number in rows known as periods and columns known as groups. Elements in the same group of the periodic table have similar chemical properties. Elements can be classified as metals, metalloids, and nonmetals, or as a main-group elements, transition metals, and inner transition metals. Groups are numbered 1–18 from left to right. The elements in group 1 are known as the alkali metals; those in group 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in 18 are the noble gases.
5.1 Atomic Mass
Learning Objectives
By the end of this section, you will be able to:
- Define the average atomic mass
- Calculate average atomic mass and isotopic abundance
Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single atom is approximately equal to its mass number (a whole number). However, the average masses of atoms of most elements are not whole numbers because most elements exist naturally as mixtures of two or more isotopes.
The mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted, average mass of all the isotopes present in a naturally occurring sample of that element. This is equal to the sum of each individual isotope’s mass multiplied by its fractional abundance.
For example, the element boron is composed of two isotopes: About 19.9% of all boron atoms are 10B with a mass of 10.0129 amu, and the remaining 80.1% are 11B with a mass of 11.0093 amu. The average atomic mass for boron is calculated to be:
It is important to understand that no single boron atom weighs exactly 10.8 amu; 10.8 amu is the average mass of all boron atoms, and individual boron atoms weigh either approximately 10 amu or 11 amu.
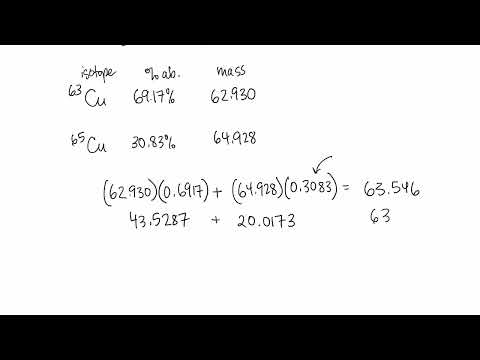 Watch on YouTube
Watch on YouTube
Calculation of Average Atomic Mass
A meteorite found in central Indiana contains traces of the noble gas neon picked up from the solar wind during the meteorite’s trip through the solar system. Analysis of a sample of the gas showed that it consisted of 91.84%
20Ne (mass 19.9924 amu), 0.47%
21Ne (mass 20.9940 amu), and 7.69%
22Ne (mass 21.9914 amu). What is the average mass of the neon in the solar wind?
Solution
The average mass of a neon atom in the solar wind is 20.15 amu. (The average mass of a terrestrial neon atom is 20.1796 amu. This result demonstrates that we may find slight differences in the natural abundance of isotopes, depending on their origin.)
Check Your Learning
A sample of magnesium is found to contain 78.70% of
24Mg atoms (mass 23.98 amu), 10.13% of
25Mg atoms (mass 24.99 amu), and 11.17% of
26Mg atoms (mass 25.98 amu). Calculate the average mass of a Mg atom.
We can also do variations of this type of calculation, as shown in the next example.
Calculation of Percent Abundance
Naturally occurring chlorine consists of
35Cl (mass 34.96885 amu) and
37Cl (mass 36.96590 amu), with an average mass of 35.453 amu. What is the percent composition of Cl in terms of these two isotopes?
Solution
The average mass of chlorine is the fraction that is
35Cl times the mass of
35Cl plus the fraction that is
37Cl times the mass of
37Cl.
If we let x represent the fraction that is 35Cl, then the fraction that is 37Cl is represented by 1.00 − x.
(The fraction that is 35Cl + the fraction that is 37Cl must add up to 1, so the fraction of 37Cl must equal 1.00 − the fraction of 35Cl.)
Substituting this into the average mass equation, we have:
So solving yields: x = 0.7576, which means that 1.00 − 0.7576 = 0.2424. Therefore, chlorine consists of 75.76% 35Cl and 24.24% 37Cl.
Check Your Learning
Naturally occurring copper consists of
63Cu (mass 62.9296 amu) and
65Cu (mass 64.9278 amu), with an average mass of 63.546 amu. What is the percent composition of Cu in terms of these two isotopes?
69.15% Cu-63 and 30.85% Cu-65
Use this simulation to make mixtures of the main isotopes of the first 18 elements, gain experience with average atomic mass, and check naturally occurring isotope ratios using the Isotopes and Atomic Mass simulation.
As you will learn, isotopes are important in nature and especially in human understanding of science and medicine. Let's consider just one natural, stable isotope: Oxygen-18, which is noted in the table above and is referred to as one of the environmental isotopes. It is important in paleoclimatology, for example, because scientists can use the ratio between Oxygen-18 and Oxygen-16 in an ice core to determine the temperature of precipitation over time. Oxygen-18 was also critical to the discovery of metabolic pathways and the mechanisms of enzymes. Mildred Cohn pioneered the usage of these isotopes to act as tracers, so that researchers could follow their path through reactions and gain a better understanding of what is happening. One of her first discoveries provided insight into the phosphorylation of glucose that takes place in mitochondria. And the methods of using isotopes for this research contributed to entire fields of study.
The occurrence and natural abundances of isotopes can be experimentally determined using an instrument called a mass spectrometer. Mass spectrometry (MS) is widely used in chemistry, forensics, medicine, environmental science, and many other fields to analyze and help identify the substances in a sample of material. In a typical mass spectrometer (Figure 5.1), the sample is vaporized and exposed to a high-energy electron beam that causes the sample’s atoms (or molecules) to become electrically charged, typically by losing one or more electrons. These cations then pass through a (variable) electric or magnetic field that deflects each cation’s path to an extent that depends on both its mass and charge (similar to how the path of a large steel ball rolling past a magnet is deflected to a lesser extent that that of a small steel ball). The ions are detected, and a plot of the relative number of ions generated versus their mass-to-charge ratios (a mass spectrum) is made. The height of each vertical feature or peak in a mass spectrum is proportional to the fraction of cations with the specified mass-to-charge ratio. Since its initial use during the development of modern atomic theory, MS has evolved to become a powerful tool for chemical analysis in a wide range of applications.
Watch this explanation of of mass spectrometry.
Watch this video from the Royal Society for Chemistry for a brief description of the rudiments of mass spectrometry.
Link to Supplemental Exercises
Supplemental exercises are available if you would like more practice with these concepts.
5.2 The Periodic Table
By the end of this section, you will be able to:
- State the periodic law and explain the organization of elements in the periodic table
- Predict the general properties of elements based on their location within the periodic table
- Identify metals, nonmetals, and metalloids by their properties and/or location on the periodic table
As early chemists worked to purify ores and discovered more elements, they realized that various elements could be grouped together by their similar chemical behaviors. One such grouping includes lithium (Li), sodium (Na), and potassium (K): These elements all are shiny, conduct heat and electricity well, and have similar chemical properties. A second grouping includes calcium (Ca), strontium (Sr), and barium (Ba), which also are shiny, good conductors of heat and electricity, and have chemical properties in common. However, the specific properties of these two groupings are notably different from each other. For example: Li, Na, and K are much more reactive than are Ca, Sr, and Ba; Li, Na, and K form compounds with oxygen in a ratio of two of their atoms to one oxygen atom, whereas Ca, Sr, and Ba form compounds with one of their atoms to one oxygen atom. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) also exhibit similar properties to each other, but these properties are drastically different from those of any of the elements above.
Dimitri Mendeleev in Russia (1869) and Lothar Meyer in Germany (1870) independently recognized that there was a periodic relationship among the properties of the elements known at that time. Both published tables with the elements arranged according to increasing atomic mass. But Mendeleev went one step further than Meyer: He used his table to predict the existence of elements that would have the properties similar to aluminum and silicon, but were yet unknown. The discoveries of gallium (1875) and germanium (1886) provided great support for Mendeleev’s work. Although Mendeleev and Meyer had a long dispute over priority, Mendeleev’s contributions to the development of the periodic table are now more widely recognized (Figure 5.2).
By the twentieth century, it became apparent that the periodic relationship involved atomic numbers rather than atomic masses. The modern statement of this relationship, the periodic law, is as follows: the properties of the elements are periodic functions of their atomic numbers. A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 5.3). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name. The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column. In the United States, the labels traditionally were numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common. For the table to fit on a single page, parts of two of the rows, a total of 14 columns, are usually written below the main body of the table.
Even after the periodic nature of elements and the table itself were widely accepted, gaps remained. Mendeleev had predicted, and others including Henry Moseley had later confirmed, that there should be elements below Manganese in Group 7. German chemists Ida Tacke and Walter Noddack set out to find the elements, a quest being pursued by scientists around the world. Their method was unique in that they did not only consider the properties of manganese, but also the elements horizontally adjacent to the missing elements 43 and 75 on the table. Thus, by investigating ores containing minerals of ruthenium (Ru), tungsten (W), osmium (Os), and so on, they were able to identify naturally occurring elements that helped complete the table. Rhenium, one of their discoveries, was one of the last natural elements to be discovered and is the last stable element to be discovered. (Francium, the last natural element to be discovered, was identified by Marguerite Perey in 1939.)
Many elements differ dramatically in their chemical and physical properties, but some elements are similar in their behaviors. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and conduct heat and electricity well. Other elements are not shiny, malleable, or ductile, and are poor conductors of heat and electricity. We can sort the elements into large classes with common properties: metals (elements that are shiny, malleable, good conductors of heat and electricity—shaded yellow); nonmetals (elements that appear dull, poor conductors of heat and electricity—shaded green); and metalloids (elements that conduct heat and electricity moderately well, and possess some properties of metals and some properties of nonmetals—shaded purple).
The elements can also be classified into the main-group elements (or representative elements) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12; 1 and inner transition metals in the two rows at the bottom of the table (the top-row elements are called lanthanides and the bottom-row elements are actinides; Figure 5.4). The elements can be subdivided further by more specific properties, such as the composition of the compounds they form. For example, the elements in group 1 (the first column) form compounds that consist of one atom of the element and one atom of hydrogen. These elements (except hydrogen) are known as alkali metals, and they all have similar chemical properties. The elements in group 2 (the second column) form compounds consisting of one atom of the element and two atoms of hydrogen: These are called alkaline earth metals, with similar properties among members of that group. Other groups with specific names are the pnictogens (group 15), chalcogens (group 16), halogens (group 17), and the noble gases (group 18, also known as inert gases). The groups can also be referred to by the first element of the group: For example, the chalcogens can be called the oxygen group or oxygen family. Hydrogen is a unique, nonmetallic element with properties similar to both group 1 and group 17 elements. For that reason, hydrogen may be shown at the top of both groups, or by itself.
Naming Groups of Elements
Atoms of each of the following elements are essential for life. Give the group name for the following elements:
(a) chlorine
(b) calcium
(c) sodium
(d) sulfur
Solution
The family names are as follows:
(a) halogen
(b) alkaline earth metal
(c) alkali metal
(d) chalcogen
Check Your Learning
Give the group name for each of the following elements:
(a) krypton
(b) selenium
(c) barium
(d) lithium
(a) noble gas; (b) chalcogen; (c) alkaline earth metal; (d) alkali metal
As you will learn in your further study of chemistry, elements in groups often behave in a somewhat similar manner. This is partly due to the number of electrons in their outer shell and their similar readiness to bond. These shared properties can have far-ranging implications in nature, science, and medicine. For example, when Gertrude Elion and George Hitchens were investigating ways to interrupt cell and virus replication to fight diseases, they utilized the similarity between sulfur and oxygen (both in Group 16) and their capacity to bond in similar ways. Elion focused on purines, which are key components of DNA and which contain oxygen. She found that by introducing sulfur-based compounds (called purine analogues) that mimic the structure of purines, molecules within DNA would bond to the analogues rather than the "regular" DNA purine. With the normal DNA bonding and structure altered, Elion successfully interrupted cell replication. At its core, the strategy worked because of the similarity between sulfur and oxygen. Her discovery led directly to important treatments for leukemia. Overall, Elion's work with George Hitchens not only led to more treatments, but also changed the entire methodology of drug development. By using specific elements and compounds to target specific aspects of tumor cells, viruses, and bacteria, they laid the groundwork for many of today's most common and important medicines, used to help millions of people each year. They were awarded the Nobel Prize in 1988.
In studying the periodic table, you might have noticed something about the atomic masses of some of the elements. Element 43 (technetium), element 61 (promethium), and most of the elements with atomic number 84 (polonium) and higher have their atomic mass given in square brackets. This is done for elements that consist entirely of unstable, radioactive isotopes (you will learn more about radioactivity in the nuclear chemistry chapter). An average atomic weight cannot be determined for these elements because their radioisotopes may vary significantly in relative abundance, depending on the source, or may not even exist in nature. The number in square brackets is the atomic mass number (and approximate atomic mass) of the most stable isotope of that element.
Link to Supplemental Exercises
Supplemental exercises are available if you would like more practice with these concepts.
Footnotes
- Per the IUPAC definition, group 12 elements are not transition metals, though they are often referred to as such. Additional details on this group's elements are provided in a chapter on transition metals and coordination chemistry.