Vapor pressure is defined as the pressure at which a gas coexists with its solid or liquid phase. This pressure can be lowered by dissolving a nonvolatile substance in a volatile liquid. Vapor pressure lowering is an example of a colligative property, as this property depends only on the concentration of solute particles. Raoult's law defines the relationship between a solution’s vapor pressure and the vapor pressures and concentrations of its components. Distillation is a selective vaporization process by which solutions whose components have significantly different vapor pressures can be separated.
4.1 Phase Transitions
Learning Objectives
By the end of this section, you will be able to:
- Explain the relation between phase transition temperatures and intermolecular attractive forces
- Describe the process of distillation and its practical applications
4.1.1 Vaporization and Condensation
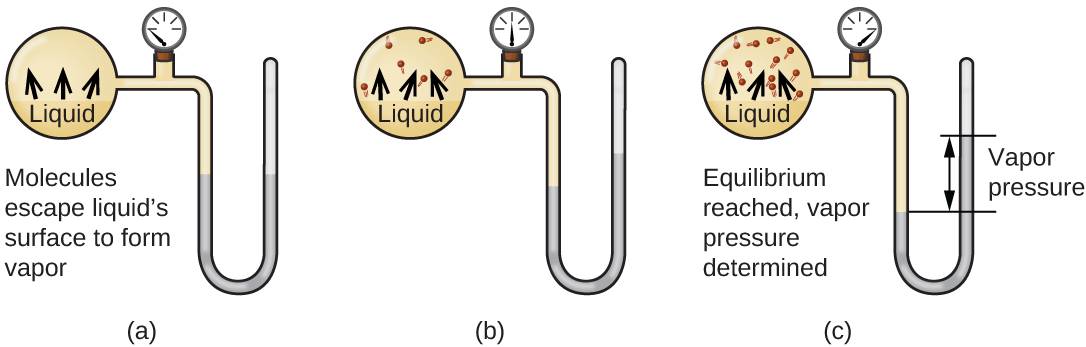
When a liquid vaporizes in a closed container, gas molecules cannot escape. As these gas phase molecules move randomly about they will occasionally collide with the surface of the condensed phase and in some cases, these collisions will result in the molecules re-entering the condensed phase. The change from the gas phase to the liquid is called condensation. When the rate of condensation becomes equal to the rate of vaporization, neither the amount of the liquid nor the amount of the vapor in the container changes. The vapor in the container is then said to be in equilibrium with the liquid. Keep in mind that this is not a static situation, as molecules are continually exchanged between the condensed and gaseous phases. Such is an example of a dynamic equilibrium, the status of a system in which reciprocal processes (for example, vaporization and condensation) occur at equal rates. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor pressure (or equilibrium vapor pressure). The area of the surface of the liquid in contact with a vapor and the size of the vessel have no effect on the vapor pressure although they do affect the time required for the equilibrium to be reached. We can measure the vapor pressure of a liquid by placing a sample in a closed container like that illustrated in Figure 4.1, and using a manometer to measure the increase in pressure that is due to the vapor in equilibrium with the condensed phase.
Figure 4.1
In a closed container, dynamic equilibrium is reached when (a) the rate of molecules escaping from the liquid to become the gas (b) increases and eventually (c) equals the rate of gas molecules entering the liquid. When this equilibrium is reached the vapor pressure of the gas is constant although the vaporization and condensation processes continue.
The chemical identities of the molecules in a liquid determine the types (and strengths) of intermolecular attractions possible; consequently, different substances will exhibit different equilibrium vapor pressures. Relatively strong intermolecular attractive forces will serve to impede vaporization as well as favoring “recapture” of gas-phase molecules when they collide with the liquid surface resulting in a relatively low vapor pressure. Weak intermolecular attractions present less of a barrier to vaporization and a reduced likelihood of gas recapture yielding relatively high vapor pressures. The following example illustrates this dependence of vapor pressure on intermolecular attractive forces.
Explaining Vapor Pressure in Terms of IMFs
Given the shown structural formulas for these four compounds, explain their relative vapor pressures in terms of types and extents of IMFs:
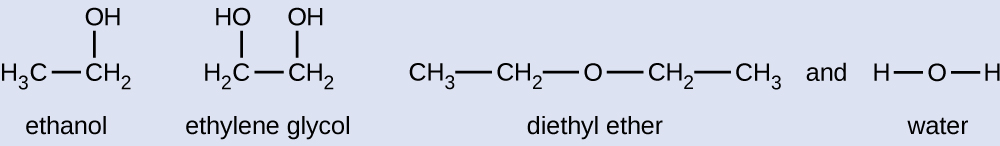
Solution
Diethyl ether has a very small dipole and most of its intermolecular attractions are London forces. Although this molecule is the largest of the four under consideration, its IMFs are the weakest and as a result, its molecules most readily escape from the liquid. It also has the highest vapor pressure. Due to its smaller size, ethanol exhibits weaker dispersion forces than diethyl ether. However, ethanol is capable of hydrogen bonding and therefore exhibits stronger overall IMFs, which means that fewer molecules escape from the liquid at any given temperature, and so ethanol has a lower vapor pressure than diethyl ether. Water is much smaller than either of the previous substances and exhibits weaker dispersion forces but its extensive hydrogen bonding provides stronger intermolecular attractions, fewer molecules escaping the liquid, and a lower vapor pressure than for either diethyl ether or ethanol. Ethylene glycol has two −OH groups so like water, it exhibits extensive hydrogen bonding. It is much larger than water and thus experiences larger London forces. Its overall IMFs are the largest of these four substances which means its vaporization rate will be the slowest and consequently, its vapor pressure the lowest.
Check Your Learning
At 20 °C, the vapor pressures of several alcohols are given in this table. Explain these vapor pressures in terms of types and extents of IMFs for these alcohols:
| Compound | methanol CH3OH | ethanol C2H5OH | propanol C3H7OH | butanol C4H9OH |
| Vapor Pressure at 20 °C | 11.9 kPa | 5.95 kPa | 2.67 kPa | 0.56 kPa |
All these compounds exhibit hydrogen bonding; these strong IMFs are difficult for the molecules to overcome, so the vapor pressures are relatively low. As the size of molecule increases from methanol to butanol, dispersion forces increase, which means that the vapor pressures decrease as observed:
Pmethanol > Pethanol > Ppropanol > Pbutanol.
As temperature increases, the vapor pressure of a liquid also increases due to the increased average KE of its molecules. Recall that at any given temperature the molecules of a substance experience a range of kinetic energies with a certain fraction of molecules having a sufficient energy to overcome IMF and escape the liquid (vaporize). At a higher temperature a greater fraction of molecules have enough energy to escape from the liquid as shown in Figure 4.2. The escape of more molecules per unit of time and the greater average speed of the molecules that escape both contribute to the higher vapor pressure.
Figure 4.2
Temperature affects the distribution of kinetic energies for the molecules in a liquid. At the higher temperature more molecules have the necessary kinetic energy, KE, to escape from the liquid into the gas phase.

The quantitative relation between a substance’s vapor pressure and its temperature is described by the Clausius-Clapeyron equation:
where ΔHvap is the enthalpy of vaporization for the liquid, R is the gas constant, and A is a constant whose value depends on the chemical identity of the substance. Temperature T must be in Kelvin in this equation. This equation is often rearranged into logarithmic form to yield the linear equation:
This linear equation may be expressed in a two-point format that is convenient for use in various computations, as demonstrated in the example exercises that follow. If at temperature T1, the vapor pressure is P1, and at temperature T2, the vapor pressure is P2, the corresponding linear equations are:
Since the constant, A, is the same, these two equations may be rearranged to isolate ln A and then set them equal to one another:
which can be combined into:
Estimating Enthalpy of Vaporization
Isooctane (2,2,4-trimethylpentane) has an octane rating of 100. It is used as one of the standards for the octane-rating system for gasoline. At 34.0 °C, the vapor pressure of isooctane is 10.0 kPa, and at 98.8 °C, its vapor pressure is 100.0 kPa. Use this information to estimate the enthalpy of vaporization for isooctane.
Solution
The enthalpy of vaporization, ΔHvap, can be determined by using the Clausius-Clapeyron equation:
Since we have two vapor pressure-temperature values (T1 = 34.0 °C = 307.2 K, P1 = 10.0 kPa and T2 = 98.8 °C = 372.0 K, P2 = 100 kPa), we can substitute them into this equation and solve for ΔHvap. Rearranging the Clausius-Clapeyron equation and solving for ΔHvap yields:
Note that the pressure can be in any units, so long as they agree for both P values but the temperature must be in kelvin for the Clausius-Clapeyron equation to be valid.
Check Your Learning
At 20.0 °C, the vapor pressure of ethanol is 5.95 kPa, and at 63.5 °C, its vapor pressure is 53.3 kPa. Use this information to estimate the enthalpy of vaporization for ethanol.
41,360 J/mol or 41.4 kJ/mol
Estimating Temperature (or Vapor Pressure)
For benzene (C6H6), the normal boiling point is 80.1 °C and the enthalpy of vaporization is 30.8 kJ/mol. What is the boiling point of benzene in Denver where atmospheric pressure = 83.4 kPa?
Solution
If the temperature and vapor pressure are known at one point, along with the enthalpy of vaporization, ΔHvap, then the temperature that corresponds to a different vapor pressure (or the vapor pressure that corresponds to a different temperature) can be determined by using the Clausius-Clapeyron equation:
Since the normal boiling point is the temperature at which the vapor pressure equals atmospheric pressure at sea level, we know one vapor pressure-temperature value (T1 = 80.1 °C = 353.3 K, P1 = 101.3 kPa, ΔHvap = 30.8 kJ/mol) and want to find the temperature (T2) that corresponds to vapor pressure P2 = 83.4 kPa. We can substitute these values into the Clausius-Clapeyron equation and then solve for T2. Rearranging the Clausius-Clapeyron equation and solving for T2 yields:
Check Your Learning
For acetone (CH3)2CO, the normal boiling point is 56.5 °C and the enthalpy of vaporization is 31.3 kJ/mol. What is the vapor pressure of acetone at 25.0 °C?
4.1.5 Enthalpy of Vaporization
Vaporization is an endothermic process. The cooling effect can be evident when you leave a swimming pool or a shower. When the water on your skin evaporates, it removes heat from your skin and causes you to feel cold. The energy change associated with the vaporization process is the enthalpy of vaporization, ΔHvap. For example, the vaporization of water at standard temperature is represented by:
As described in the chapter on thermochemistry, the reverse of an endothermic process is exothermic. And so, the condensation of a gas releases heat:
EXAMPLE 4.1.6
Using Enthalpy of Vaporization
One way our body is cooled is by evaporation of the water in sweat (Figure 4.3 ). In very hot climates we can lose as much as 1.5 L of sweat per day. Although sweat is not pure water, we can get an approximate value of the amount of heat removed by evaporation by assuming that it is. How much heat is required to evaporate 1.5 L of water (1.5 kg) at T = 37 °C (normal body temperature); ΔH vap = 43.46 kJ/mol at 37 °C.
Figure 4.3
Evaporation of sweat helps cool the body. (credit: “Kullez”/Flickr)

Solution
We start with the known volume of sweat (approximated as just water) and use the given information to convert to the amount of heat needed:
Thus, 3600 kJ of heat are removed by the evaporation of 1.5 L of water.
Check Your Learning
How much heat is required to evaporate 100.0 g of liquid ammonia, NH3, at its boiling point if its enthalpy of vaporization is 4.8 kJ/mol?
4.1.7 Vapor Pressure Lowering
As described in the chapter on liquids and solids, the equilibrium vapor pressure of a liquid is the pressure exerted by its gaseous phase when vaporization and condensation are occurring at equal rates:
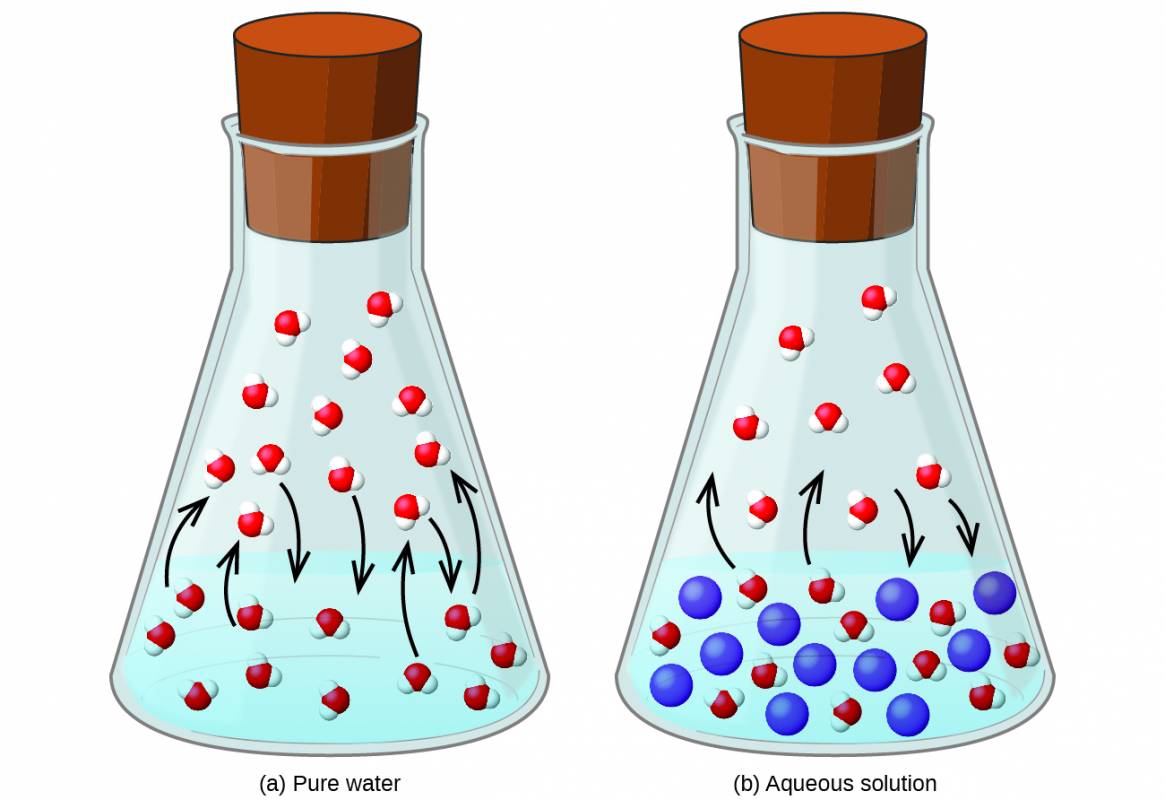
Dissolving a nonvolatile substance in a volatile liquid results in a lowering of the liquid’s vapor pressure. This phenomenon can be rationalized by considering the effect of added solute molecules on the liquid's vaporization and condensation processes. To vaporize, solvent molecules must be present at the surface of the solution. The presence of solute decreases the surface area available to solvent molecules and thereby reduces the rate of solvent vaporization. Since the rate of condensation is unaffected by the presence of solute, the net result is that the vaporization-condensation equilibrium is achieved with fewer solvent molecules in the vapor phase (i.e., at a lower vapor pressure) (Figure 4.4). While this interpretation is useful, it does not account for several important aspects of the colligative nature of vapor pressure lowering. A more rigorous explanation involves the property of entropy, a topic of discussion in a later text chapter on thermodynamics. For purposes of understanding the lowering of a liquid's vapor pressure, it is adequate to note that the more dispersed nature of matter in a solution, compared to separate solvent and solute phases serves to effectively stabilize the solvent molecules and hinder their vaporization. A lower vapor pressure results, and a correspondingly higher boiling point as described in the next section of this module.
Figure 4.4
The presence of nonvolatile solutes lowers the vapor pressure of a solution by impeding the evaporation of solvent molecules.
The relationship between the vapor pressures of solution components and the concentrations of those components is described by Raoult’s law: The partial pressure exerted by any component of an ideal solution is equal to the vapor pressure of the pure component multiplied by its mole fraction in the solution.
where PA is the partial pressure exerted by component A in the solution, \(\(\)\) is the vapor pressure of pure A, and XA is the mole fraction of A in the solution.
Recalling that the total pressure of a gaseous mixture is equal to the sum of partial pressures for all its components (Dalton’s law of partial pressures), the total vapor pressure exerted by a solution containing i components is
A nonvolatile substance is one whose vapor pressure is negligible (P* ≈ 0), and so the vapor pressure above a solution containing only nonvolatile solutes is due only to the solvent:
EXAMPLE 4.1.8
Calculation of a Vapor Pressure
Compute the vapor pressure of an ideal solution containing 92.1 g of glycerin, C3H5(OH)3, and 184.4 g of ethanol, C2H5OH, at 40 °C. The vapor pressure of pure ethanol is 0.178 atm at 40 °C. Glycerin is essentially nonvolatile at this temperature.
Solution
Since the solvent is the only volatile component of this solution, its vapor pressure may be computed per Raoult’s law as:
First, calculate the molar amounts of each solution component using the provided mass data.
Next, calculate the mole fraction of the solvent (ethanol) and use Raoult’s law to compute the solution’s vapor pressure.
Check Your Learning
A solution contains 5.00 g of urea, CO(NH2)2 (a nonvolatile solute) and 0.100 kg of water. If the vapor pressure of pure water at 25 °C is 23.7 torr, what is the vapor pressure of the solution assuming ideal behavior?
4.1.9 Distillation of Solutions
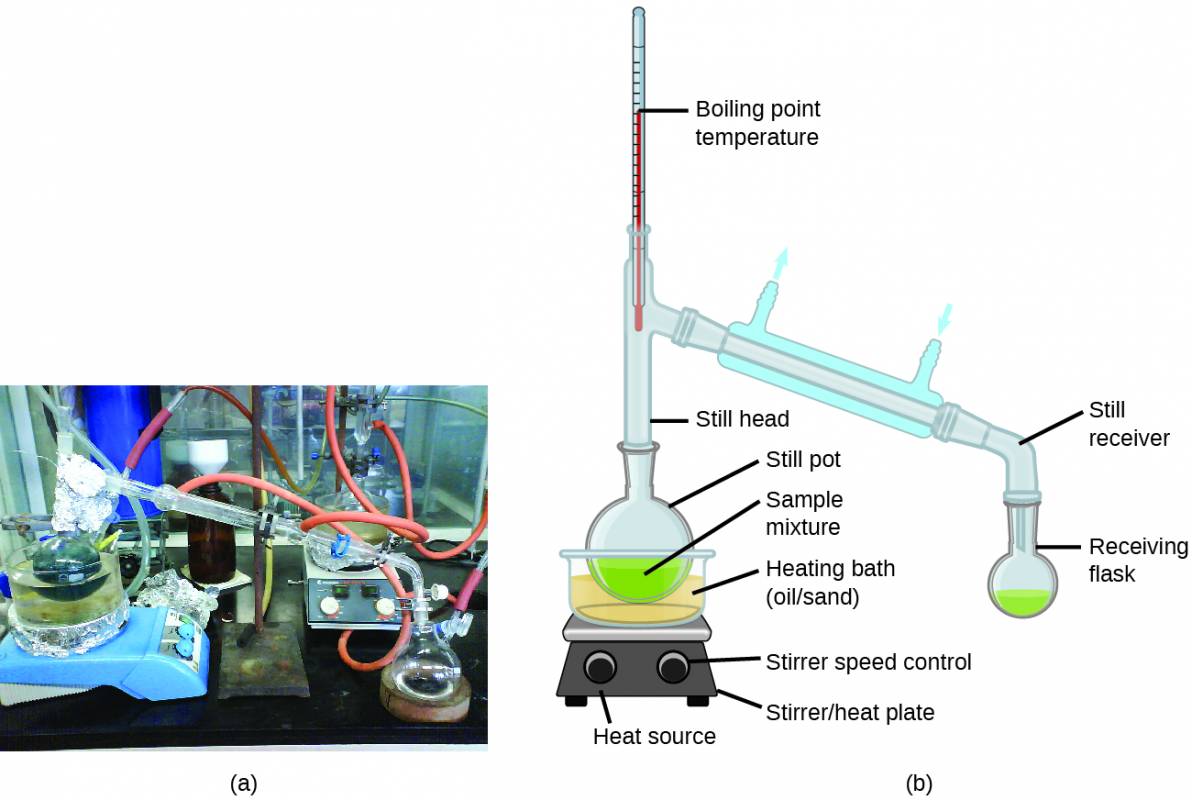
Solutions whose components have significantly different vapor pressures may be separated by a selective vaporization process known as distillation. Consider the simple case of a mixture of two volatile liquids A and B, with A being the more volatile liquid. Raoult’s law can be used to show that the vapor above the solution is enriched in component A, that is, the mole fraction of A in the vapor is greater than the mole fraction of A in the liquid (see end-of-chapter Exercise 65). By appropriately heating the mixture, component A may be vaporized, condensed, and collected—effectively separating it from component B.
Distillation is widely applied in both laboratory and industrial settings, being used to refine petroleum, to isolate fermentation products, and to purify water. A typical apparatus for laboratory-scale distillations is shown in Figure 4.5.
Figure 4.5
A typical laboratory distillation unit is shown in (a) a photograph and (b) a schematic diagram of the components. (credit a: modification of work by “Rifleman82”/Wikimedia commons; credit b: modification of work by “Slashme”/Wikimedia Commons)
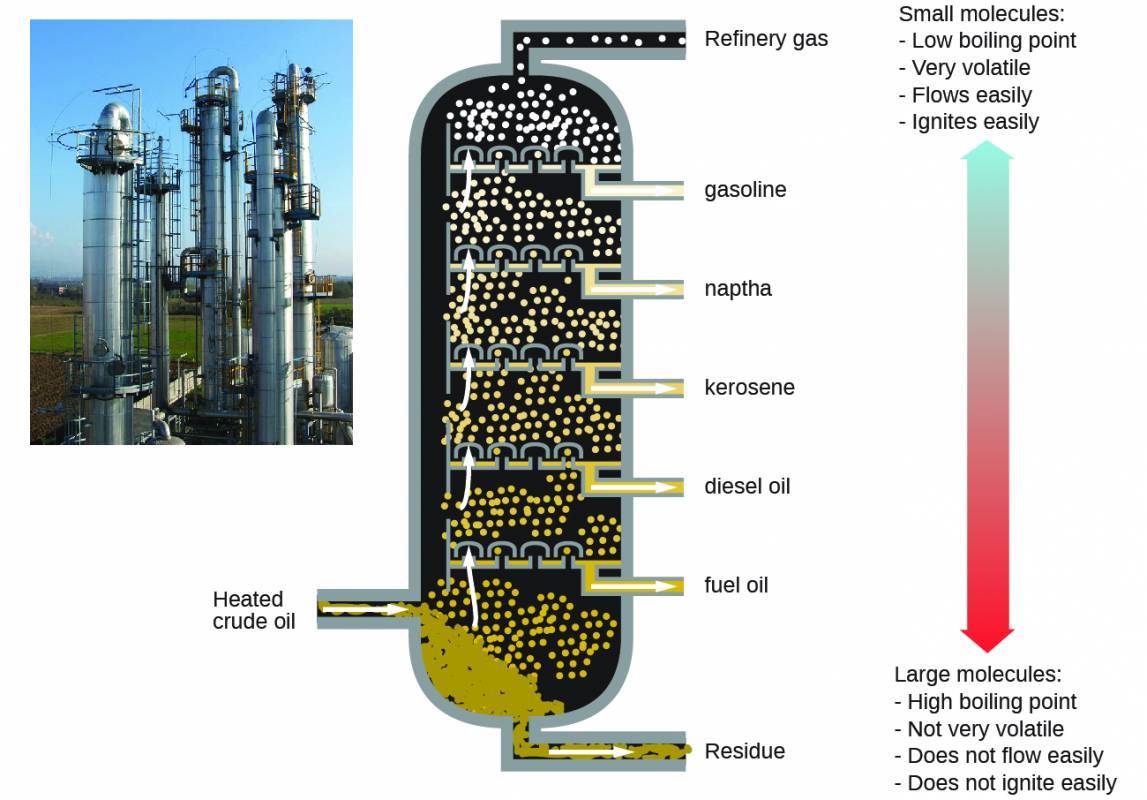
Oil refineries use large-scale fractional distillation to separate the components of crude oil. The crude oil is heated to high temperatures at the base of a tall fractionating column vaporizing many of the components that rise within the column. As vaporized components reach adequately cool zones during their ascent, they condense and are collected. The collected liquids are simpler mixtures of hydrocarbons and other petroleum compounds that are of appropriate composition for various applications (e.g., diesel fuel, kerosene, gasoline), as depicted in Figure 4.6.
Figure 4.6
Crude oil is a complex mixture that is separated by large-scale fractional distillation to isolate various simpler mixtures.